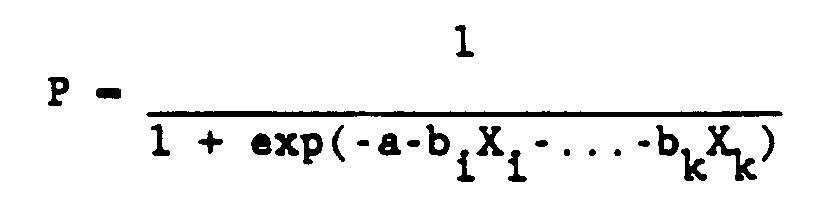
CRAIG EBERT[2], SARAH FOSTER[2], MICHAEL J. GIBBS[2], KEVIN HEARLE[2], BRIAN HICKS[2], PATSY H. LILL[3], JANICE LONGSTRETH[4], NEIL PATEL[1], HUGH M. PITCHER[1], ALAN F. TERAMURA[5], DENNIS TIRPAK[1], JIM TITUS[1], JOHN B. WELLS[6], G. Z. WHITTEN[7], ROBERT WORREST[8]
1 U.S. ENVIRONMENTAL PROTECTION AGENCY, 401 M STREET, S.W., WASHINGTON, DC
2 ICF INCORPORATED, 9300 LEE HIGHWAY, FAIRFAX, VA
3 UNIVERSITY OF SOUTH CAROLINA SCHOOL OF MEDICINE, COLUMBIA, SC
4 ICF CLEMENT, 9300 LEE HIGHWAY, FAIRFAX, VA
5 DEPARTMENT OF BOTANY, UNIVERSITY OF MARYLAND, COLLEGE PARK, MD
6 THE BRUCE COMPANY, WASHINGTON, DC
7 SYSTEMS APPLICATIONS, INC., 101 LUCAS VALLEY ROAD, SAN RAFAEL, CA
8 CORVALLIS ENVIRONMENTAL RESEARCH LABORATORY, 200 SOUTHWEST 35TH STREET, CORVALLIS, OR
DECEMBER 1987
CATARACTS AND OTHER EYE DISORDERS
SUMMARY
Cataracts are opacities that develop in the lens of the eye and impair vision. In the United States and other developed countries, cataract operations prevent most cataracts from causing blindness. However, in the U.S. cataract remains the third leading cause of legal blindness. In developing countries where such operations are not always available, cataracts often result in blindness.
Scientific understanding of the physical mechanisms which cause cataracts is incomplete; it is likely that more than one mechanism operates. Epidemiological studies, laboratory animal studies, and biochemical analysis support the belief that some cataracts are caused by ultraviolet radiation B (UV-B). Ultraviolet radiation A and other causes are also likely. A change in the amount of UV-B radiation is reasonably likely to alter the incidence of cataracts. UV-B may also play a role in causing or exacerbating other eye disorders. Ozone modification that alters the amount of UV-B reaching the earth's surface is likely to change the prevalence (and incidence) of cataracts.
FINDINGS
1. THERE APPEARS TO BE A REASONABLE PROBABILITY THAT CATARACT INCIDENCE WILL CHANGE WITH ALTERATIONS IN THE FLUX OF UV-B CAUSED BY OZONE MODIFICATION.
1a) Many possible mechanisms exist for formation of cataracts. UV-B may play an important role in some mechanisms.1b) Although the cornea and aqueous of the human eye screen out significant amounts of UV-A and UV-B radiation, nearly 50 percent of radiation at 320 nm is transmitted through to the lens. Transmittance declines substantially below 320 nm, so that less than one percent is transmitted below approximately 290 to 300 nm. However, the results of laboratory experiments on animals indicate that short wavelength UV-B (i.e., below 290 nm) is perhaps 250 times more effective than long wavelength UV-B (i.e., 320 nm) in inducing cataract.
1c) In laboratory animal experiments, the action spectrum for cataracts is weighted heavily in the UV-B range.
1d) Human cataract prevalence appears to vary with latitude and UV radiation; brunescent nuclear cataracts show the strongest relationship.
2. INCREASES IN THE AMOUNT OF UV-B THAT CAN REACH THE RETINA APPEAR CAPABLE OF CAUSING STABLE RETINAL DISORDERS AND RETINAL DEGENERATION, TWO CAUSES OF BLINDNESS.
3. UV-B MAY PLAY A ROLE IN DISORDERS OF THE EYES AS WELL AS IN DEVELOPMENTAL DISORDERS.
The longest standing hypothesis which may account for the development of senile cataracts is that radiant energy, particularly sunlight, is a major factor in the etiology of the disease. This concept apparently originated from observations reported by a number of individuals indicating that cataracts occurred more frequently or earlier in persons whose occupations kept them outdoors or that populations living in areas with longer hours of sunshine have a higher frequency of cataract than populations from areas where there is less sunshine while the early studies were severely flawed by their failure to consider adequately the possible effects of a variety of socioeconomic and other variables, Duke-Elder (1926, 1972) proposed that "the fundamental cause of cataract in all its forms may be traced to the incidence of radiant energy directly on the lens itself."
Definition of Cataract
Cataract is defined as an opacity in the normally transparent lens of the eye which produces an impairment of vision. Cataracts may occur as a result of a wide variety of factors including metabolic disorders, espouse to toxic agents, trauma, exposure to radiation, and hereditary factors. The great majority of cataracts, however, are the so-called senile cataracts which occur in older individuals and for which no specific causative factor can be identified. Cataract is a major cause of visual impairment and blindness particularly in developing countries where access to modern surgical facilities is limited. Even in the United States, cataract is the third leading cause of legal blindness. Some 60 percent of people aged 60 to 74 have at least some cataractous changes in their lenses. The only efficacious treatment for cataract at present is the surgical removal of the opaque lens and nearly 660,000 such operations were performed in 1982 in the U.S.
It is clear therefore that senile cataract is a very significant health problem, both in terms of its impact on the affected individuals and on society at large. While considerable progress has been made in elucidating the biochemical etiology of certain specific cataract types, such as sugar cataracts, there is little conclusive data on the causes of senile cataracts. It is likely that there are a variety of potential risk factors and that, in general, senile cataracts have a multifactorial etiology. This conclusion is supported by the great variability observed clinically in the time of onset, the rate of maturation, and the morphological appearance and location within the lens of these opacities. It appears that many, if not all, of the processes contributing to senile cataractogenesis are normal aging processes which for whatever reason are accelerated in certain individuals
Cataract Classification
There are now rather sophisticated systems for the classification of cataracts, Chylack et al (1978, 1983) have devised an in vitro system which is based on photographic documentation of opacification and nuclear color. Almost 2500 cataracts have been studied and classified since the original methods were adopted (Chylack et al, 1983). In addition, Marcantonio et al. (1980) have suggested a system of classification of human senile cataracts by using photography for in vivo and in vitro analysis and determining the sodium and protein content of the extracted lens. The photographic method gave consistent involvement of the lens nucleus but it was not always possible to relate sodium changes to light scattering. Marcantonio et al (1980) argue that a dual classification system is necessary because most cataracts are mixed in nature and the osmotic and nuclear mechanisms provide quite different changes in protein distribution.
The present state of knowledge concerning the human senile cataract, probably justifies a classification into only two major types in spite of the elegant classification systems mentioned above. The first and most common type of human senile cataract is the cortical cataract. Cortical cataracts are characterized by imbalances in cation levels within the lens cells which produce osmotic swelling and ultimate opacity in the lens cortex. Altered cation balance may result from damage to the NA[+], K[+]-ATPase or from compromise of the normal permeability characteristics of the lens membranes. Any of a great variety of potential insults could be the ultimate cause of such cataracts. The second major type of senile cataract is the nuclear cataract which is characterized primarily by very marked modifications to the structural proteins of the lens, the crystalline, in the central region of the lens. These two types of cataracts are not at all mutually exclusive; in many instances, senile cataracts contain both cortical and nuclear opacities. It is advisable for those who are involved in cataract research to become knowledgeable and use a recommended classification system.
Epidemiological Studies
A few general statements should be made relative to the cataract epidemiological studies. Most epidemiological studies have reported an increase in the prevalence of cataracts with an increase in age and at 65 years of age and above there is an acceleration in the prevalence of cataracts. Women have shown a larger prevalence of cataracts than men; however, this difference has not been shown to be statistically significant.
In recent years there have been a number of epidemiological studies reported which have attempted to establish that there is an association between cataract prevalence and exposure to sunlight or the ultraviolet component of sunlight. Hiller, Giacometti and Yuen (1977) utilized data on the populations sampled in two independent health surveys, the Model Reporting Area for Blindness Statistics (MRA) and the National Health and Nutrition Examination Survey (HANES) to compare the prevalence of cataract in geographical areas with varying total annual hours of sunlight. The total annual hours of sunlight was obtained from U.S. Weather Bureau statistics and ranged from 1800 to 3800 hours. For each health survey ocular diseases other than cataract cases to control cases for areas of differing total annual insolation. The findings suggest that in the youngest age group studies (20-44 yrs) there is no greater incidence of cataract in areas of high annual sunlight, but that with increasing age there appears to develop an increasing association of cataract with sunlight exposure. In the 65-74 yr sample population, there was at least a doubling of the ratio of cataract to each control disease between the lowest and highest sunshine area and in persons 75 years and older this trend was even more pronounced. The study did not consider the possible effects of genetic or socioeconomic differences among the populations from different areas nor did it consider the actual sunlight exposure of the individuals (e.g. indoor vs outdoor occupation).
Zigman, Datiles, and Torczynski (1979) have studied populations from three widely separated areas (Manila, the Philippines; Tampa, Florida; and Rochester, New York) which differ considerably in the yearly levels of ultraviolet radiation in sunlight. This study considered not only the age of the individuals studied, but also considered separately those with indoor and outdoor occupations. The findings which were based on study of extracted cataracts included analysis of the cataract type for each lens. The results indicated that no correlation existed between geographic location and distribution of cataracts except for the brunescent cataracts; i.e., those nuclear cataracts with significantly increased pigmentation levels. In Manila, the area with greatest UV, such cataracts accounted for 43% of total cataracts while in Tampa 20% of cataracts were brunescent and in Rochester, the area of least UV, only 9%. In all three populations, the percentage of brunescent cataracts extracted in persons with outdoor occupation was markedly higher than in those persons working indoors. Thus, both the latitudinal variation and the individual differences within geographic regions suggested a strong relationship between UV exposure an brunescent cataract. These data are consistent with numerous reports that tropical areas have higher cataract incidence than areas at higher latitudes and that the percentage of brunescent cataracts is higher in tropical areas (Pirie 1972 and references in Taylor 1980). The confounding factors which were pointed out by Zigman, Datiles, and Torczynski (1979) as not being controlled in their study included the economic, nutritional and genetic backgrounds of the individuals in the respective populations. While these differences would probably be substantial, particularly between Manila and the two U.S. sites, this study is significant in that it involved some biochemical evaluation of the cataracts as well as the epidemiological data.
Two recent epidemiological studies have concerned cataracts in Australian aborigines, a rural population exposed to relatively intense solar radiation. Taylor (1980) studied 350 individuals from which detailed personal histories were obtained. The possible role of a variety of personal and environmental factors in cataractogenesis were investigated. Among the 350 individuals, all of whom were over 30 years of age, 116 had lens opacities as determined by slit-lamp examination. The major findings relative to the possible influence of radiation exposure to cataractogenesis were a trend toward association of cataracts with increased hours of sunlight and with higher annual mean UV-B levels. These trends were reflected by a strong association of cataract with lower latitudes. No other environmental factors studies appeared to be associated with cataract nor did any personal factors other than age. No correlation would found between occupation and cataract; it is not clear whether this finding is inconsistent with Zigman, Datiles, and Torczynski (1979) since it is not reported whether any of the individuals studied had indoor occupations. Unfortunately, the types of cataracts present were not reported in this study (Taylor 1980).
In a second study involving a much greater geographical area and over 50% (64,307) of the total Aborigine population, Hollows and Moral (1981) also found a statistically significant correlation between environmental UV irradiation and the prevalence of cataract (p<0.005). Additionally cataracts occurred much more frequently in the younger age group (40-59 yrs) in the geographical zones of high UV radiation than in zones of low UV irradiation. The authors suggest that the Aborigines from remote rural Australia are a suitable population for such studies since their lifestyle tends to be highly uniform throughout the country. In contrast to these results, a large sample of non-Aboriginal people from the same areas showed much lower levels of cataract, particularly at younger ages, and there was no correlation between cataract prevalence and sunlight in this group. This was attributed to the much higher standard of living and the greater likelihood of indoor occupations in this group.
The data of Hollows and Moran (1981) may be sued to estimate the number of senile cataracts which are caused by sunlight if we can accept the confounding factors mentioned above. Exhibit 10-1 presents the prevalence of cataracts found in aborigines by UV zone for three different age groups. Exhibit 10-2 compares the prevalence of cataracts found in non-aborigines and aborigines who live in the same regions.
Exhibit 10-1 shows that 13.6% of the aborigines in the lowest solar region develop cataracts while about 30% in the more solar intense zones develop cataracts. These data appear to indicate that about 15% of the senile cataracts are due to solar radiation. More recently, Weale (1982) has presented a method of estimating the risk attributed to light for the incidence of senile cataracts and reported a risk factor of 5. If the risk factor may be expressed in percent form or 20%, the results are not too different from the above. It is interesting that the two sets of diverse data present such interesting results. Incidentally, the 18.3% prevalence for cataracts for the non-aborigine compared quite favorably to the Framingham Study (Kahn et al. 1977a and b) and the Gisborne Study (Martinez et al. 1982).
Thus there appears to be a consensus from these epidemiological studies in support of the notion that senile cataract or at least a particular segment of the heterogeneous mixture of opacity types which comprise senile cataracts, is associated with higher exposure to sunlight. Substantive questions could certainly be raised concerning each of the studies cited above; however, taken in aggregate they represent a variety of approaches, using different types of populations, different criteria for cataract, different sampling and statistical methods, and different variables to test the same hypothesis. It is striking that the general conclusions of each study are so similar.
Ocular Transmittance
The effect of nonionizing radiation on a cell depends on the specific chemical composition within the cell, that is, on the presence of absorbing molecules or chromophores (Lerman 1980a). This type of radiation must be absorbed in order to cause a change in the molecule since absorbed energy is required to promote a chemical change. Molecules in excited electronic states have different chemical and physical properties than their counterparts in the ground state (prior to absorption of energy). Thus cells that do not contain chemical compounds absorbing at certain wavelengths will transmit these wavelengths. For example, the nucleic acids and most proteins in a cell are essentially transparent to and completely transmit visible light but absorb certain wavelengths in the UV region (between 250 and 295 nm) and can be damaged by this form of radiation, while other macromolecules in a cell such as rhodopsin, which absorbs at 498 nm, and hemoglobin, which has absorption peaks in the UV and visible region (275, 400, and 540 to 576 nm), appear colored since they absorb visible light. These latter macromolecules can be damaged by high intensities of visible radiation at their specific absorption wavelengths.
The transmittance data for the rabbit, primate and human eye and ocular media for the wavelength range of 200nm to 2500nm are given in Exhibits 10-3, 10-4 and 10-5. Exhibits 10-6 and 10-7 present transmittance data for the 200 to 400 wavelength range (Kinsey 1948; Boettner and Wolter 1962; Maher 1978; Barker 1979).
The Action Spectrum
Since the absorption of nonionizing radiation is determined by the chemical composition of the tissue being exposed, the more radiation that the molecules absorb the greater will be the effect of the radiation. The term "action spectrum" is used as a measure of the relative effect of different wavelengths of radiation on a chemical compound, macromolecule, cell, or entire organism. The action spectrum is a plot of the dose or radiant exposure necessary to produce the defined effect versus the wavelength. For example, the maximum efficiency for experimental photokeratitis has been shown to occur at approximately 300 nm, with a smaller peak at 295 and 320 nm (Pitts 1978 and Pitts and Cullen 1981). The action spectra for photokeratitis, cataracts and retinal lesions for the rabbit, primate and human are presented in Exhibits 10-8 and 10-9.
The eye is the only organ or tissue in the body (aside from the skin) that is particularly sensitive to the non-ionizing wavelengths of radiation (longer than 280 nm) normally present in our environment. In addition to infrared and visible radiation, we are constantly exposed to ultraviolet radiation (solar and man-made) throughout life. It is estimated that approximately 8% (11 mW/cm2) of solar radiation above the atmosphere is in the ultraviolet region (280-400 nm) At sea level this is decreased to 2-5 mW/cm2, depending on geographic location and season (Lerman 1980b).
Nature has provided us with transparent ocular media which are essentially avascular and contain very few visible wavelength absorbing chromophores in order to effectively transmit (as well as refract) the specific wavelengths required to initiate the visual process by photochemical reactions. However, these tissues do have the ability to absorb varying amounts of ultraviolet radiation (particularly the ocular lens). The shorter the wavelengths of radiation absorbed the greater the potential for photic damage since there is an inverse relationship between a wavelength and the photon energy association with it. Thus, UV radiation is the non-ionizing portion of the electromagnetic spectrum which could cause the most damage, provided that it is absorbed. This axiom applies to all the ocular tissues including the retina in the very young eye where the lens has not as yet become as effective UV filter but, in particular, the ocular lens sustains the greatest amount of photochemical change during a lifetime of exposure to ambient UV radiation.
Some Biochemical Mechanisms
There are several mechanisms which are biochemically related to radiation damage to the eye and the lens in particular. These mechanisms include photo-oxidation of free and protein bound tryptophan, photosynthesis processes involving the activated species of oxygen, disruption of the cation transport system and damage to the nucleic acids (DNA) of the lens epithelium. Some of these mechanisms have only been studied only in cultures, some in vitro and others in vivo It is important to be able to protect the mechanism to a real live situation in order to be able to evaluate the relative importance of the factors involved in inducing the senile cataract. Prior to discussing these mechanisms it may be desirable to review some of the basic concepts in the biochemical mechanisms of radiation induced damage; therefore, some current interpretations of lens free radicals and oxidation reduction reactions relative to cataract follows. Exposure to UV radiation initiates enzymatic activity involved in cellular protection from oxidative processes which may be due to both light and metabolism (Exhibit 10-10) Catalase destroys hydrogen peroxide, which is produced in all cells by metabolism; superoxide dismutase (SOD) has the responsibility of destroying the superoxide radical, a very toxic radical due to its powerful oxidative nature. In the lens cortex, there is very little SOD or catalase, but they are highly concentrated in the epithelial cells. Of all the ocular tissues, the greatest concentrations of these enzymes are present in the retina. In the lens cortex there are other agents to protect against oxidation such as glutathione (GSH) and ascorbic acid. There is only a small amount of vitamin E in the lens, but attempting to protect animals from ocular tissue oxidative damage by feeding them high levels of vitamin E or other anti-oxidants has not succeeded. The sum total of all of the anti-oxidants that are present in the ocular tissues still does not protect against the formation of free radical oxidative reactions to near-UV radiation. Another toxic oxidant that can form is singlet oxygen.
The content of the stable free radicals previously described is highest in the cortex and seems to decrease in the nucleus, of the lens. The process of aggregation of soluble proteins (TSP) occurs both in the cortex and in the nucleus. Because of the conservative growth process of the lens, much of the aggregated material remains in the nucleus indefinitely and often in a form that is associated with the fiber cell membranes. The lens fraction called the insoluble fraction contains both membranes and the aggregated proteins. There is a chemical reaction between the free radicals and proteins so that the protein binds to free radicals chemically, which quenches the ESR (electron spin resonance) signal. Multiple molecular species of proteins can be associated through new crosslinks leading to aggregation and, therefore, light scattering. In the nucleus of the lens, the free radicals are quenched by their reactions with proteins. Since all enzymes are proteins, this may illustrate a universal process whereby enzyme activity inhibited by near-UV radiation. These changes would eventually cause malfunction of all cells and tissues.
Exhibit 10-11 shows a scheme of a series of enzymatic oxidation: reduction reactions going on in all cells that are influenced by aging and exposure to near-ultraviolet radiation. These reactions are all linked together. The co-factor NADH/NADP system is linked to the oxidation and reduction of glutathione (GSH). When oxidation occurs, reduced GSH becomes oxidized GSSG, changes which also relate to protein oxidation involving SS-crosslinks. Many enzymes, in order to remain active, must maintain sulfhydryl groups (SH) of cysteine in reduced form in order for the protein portion to maintain its activities as an enzyme. Many enzymes require free-SH groups in their active sites to retain their activity. If these enzymes are oxidized so that disulfides form, they are inactivated. For example, if high concentrations of hydrogen peroxide are generated, protein SH will convert to SS thereby inhibiting the activity. Several of the enzymes referred to in Exhibit 10-11 are known to be reduced with enhanced aging in most living cells. In many cases the cells turn over, so that cells' enzymatic activities are maintained, but where there are populations of cells that do not turn over very actively, a cumulative process can lead to a great loss of enzyme activities. This may occur in the lens.
One enzyme that is diminished with aging is glutathione reductase and it also diminishes under in vitro circumstances in living tissues when near-UV radiation is provided in the presence of excess tryptophan. There has not yet been an aging or photoinactivation study of glutathione peroxidase. The SOD is very important as a protective agent because it destroys the very toxic superoxide radical. SOD activity does diminish with aging, but is not sensitive to destruction from near-VU radiation (with tryptophan as a sensitizer). Because the lens substance contains little oxygen to serve as a superoxide source, the site of lens damage would be the epithelium if SOD activity were to be diminished drastically. Damage to the lens epithelium would certainly lead to both physiological and developmental anomalies in the lens and cataract. The SOD activity must be maintained in the retina, however, due to its high oxygen tension and the potential for oxidation to occur more readily.
Hydrogen peroxide (H2O2) is another very powerful oxidant that is present in ocular tissues and fluids by virtue of the action of SOD on superoxide and other metabolic systems, such as the glutathione and ascorbic acid cycles. Recently, high levels of H2O2 (mM concentrations) have been observed in the human aqueous humor by Garner and Spector (1980). This concentration of H2O2 is suspected of being capable of causing cataracts by poisoning important enzymes and by crosslinking proteins (Spector and Garner 1982). In eyes without lenses, H2O2 from the aqueous humor could more easily diffuse even to the retina (Aigman 1981, Kramer 1980), a highly hazardous circumstance. Ocular tissues utilize the enzyme catalase to destroy and detoxify H2O2. Since this enzyme is diminished with aging and has been shown to be inhibited by near-UV radiation plus tryptophan (Zigman, Yulo, and Griess 1976), it s activity loss would allow heightened levels of H2O2 to damage ocular tissue. A large imbalance in favor of H2O2 accumulation would lead to extensively altered ocular tissue proteins and much enzyme inactivation.
Glutathione reductase is another enzyme of great functional importance to the lens and retina, and it is diminished with aging and UV exposure (Kalustian, Sun and Zigman 1978). Its loss of activity would estimate oxidation of small molecules and proteins in most ocular tissues.
Another enzyme (not involved in oxidation: reduction, but significant with regard to its photoinactivation) is Na[+]K[+] ATPase. This enzyme is also sensitive to near-UV radiation in the presence of tryptophan and loses its activity accordingly. In the lens, it maintains the osmotic balance by controlling the sodium and potassium cation exchange and prevents water inhibition and swelling. In some genetic cataracts in mice, it has been found that the cause of cataract is the high concentration of an inhibitor of ATPase in the lens (Kinoshita 1974). It is likely then that a strong inhibition of lens epithelial cell ATPase by light-sensitized action could be a major factor in cataract formation osmotically. In the retina, the ATPase activity is very important in terms of the chemical reactions that support the visual process. Should this enzyme be photochemically inhibited in the retina, the functioning of the visual process would be markedly reduced. Regional Na[+]K[+] ATPase is also sensitive to inhibition by near-UV plus tryptophan.
Before discussing the biochemical studies which pertain to the possible cataractogenic effects of optical radiation, it may be pertinent to review some contrasts between human lenses and those of the most commonly used laboratory animals. All vertebrate lenses are composed essentially of a single cell type, the lens fiber, which differentiates from a single layer of epithelial cells present only at the bow or equator of the lens. New fibers are continually laid down at the periphery; thus, the oldest tissue is located at the center or nucleus of the lens and the cells become progressively younger as one moves from the center toward the lens capsule in the cortex. Cells are never sloughed form the lens and this makes the cells in the lens nucleus as old as the animal. Furthermore, differentiated lens fibers gradually lose virtually all cell organelles, including nuclei, and lose the capacity to synthesize protein as they age and are forced toward the nucleus. Therefore, the proteins, primarily lens crystallins, present in the aging human lens may be the longest-lived proteins in the organism. This means that unlike most other tissues the central portion of the lens does not have the ability to replace damaged proteins with newly synthesize proteins. This may be an important factor in the presumptive long-term effects of chronic exposure to near UV radiation. There are very clear differences between the lens nucleus and cortex in terms of their biochemistry and the loss of the capacity to repair or replace damaged proteins probably accounts for much of the difference.
There is at least one very significant difference between human lenses and the common laboratory animals and that is the presence of pigmented compounds in the human lens. While the lenses of most non-primate mammals have no pigment, the lenses of diurnal primates and a few other strongly diurnal species are yellow. Cooper and Robson (1969) demonstrated that in the human lens there are two classes of pigmented compounds. One group that is present even before birth is of low molecular weight, is water soluble and absorbs maximally at about 365 nm. A second class of colored compounds appears later, increases with age, is bound to the lens proteins and absorbs maximally near 320 nm. This latter class of chromophores is localized primarily in the lens nucleus and may be responsible for the age-related increase in pigmentation in human lenses. Since these chromophores absorb in the near UV, they may be major determinants of the effects of such radiation on the human lens.
Studies at the Biochemical Level
Studies at the biochemical level have generally been concerned with the structural modifications which crystallins undergo during aging and cataractogenesis and have attempted to explain these reactions in terms of photo-oxidative mechanisms. It is well-documented that crystallins, particularly in the lens nucleus, accumulate a variety of modifications. These include the formation of disulfides and other covalent crosslinks, the development of a novel blue fluorescence, progressive pigmentation, oxidation of methionine, racemization of aspartate residues, polypeptide chain cleavages, deamidations, aggregation and ultimate insolubilization. The lack of turnover of protein in the lens nucleus accounts for the accumulation of these modifications and they have been the subject of several recent reviews (Zigler and Goosey 1981; Hoenders and Bloemendal 1981; Harding 1981). It is clear that most of the protein changes are the result of oxidative stress, and the possible role of radiation in that stress has been the subject of much study over the last 15 years.
Pirie (1968, 1972) studied the effects of sunlight on solutions of lens proteins and of other proteins. The proteins became brown following irradiation and analysis of absorbance changes indicated similarity to those found in lens proteins from cataracts. Pirie also found decreased levels of the oxidation sensitive amino acids, histidine and tryptophan in protein free cataracts relative to normal lenses. Although the data for tryptophan was subsequently retracted as an artifact, Dilley and Pirie (1974) suggested that photooxidation of tryptophan residues, perhaps with formation of N'-formyl kynurenine, was a primary step in cataractogenesis. These studies stimulated a number of other investigators.
Kurzel (1973) performed fluorescence and phosphorescence measurements and Weiter and Finch (1975) ESR studies on human lenses and found signals which they believed to be due to tryptophan free radical species in lens proteins. Van Heyningen (1971) was able to identify several of the components contributing to the color of human lenses as kynurenine derivatives, species which can be derived from tryptophan either metabolically or photooxidatively. These were part of the low molecular weight colored material present in human lenses and Van Heyningen (1973) subsequently showed that lens proteins exposed to sunlight in the presence of these compounds were photo-oxidized more extensively than in their absence. The mechanisms of this accelerated photo-oxidation was not determined.
In addition to oxidation of protein bound tryptophan other possible mechanisms were explored. Zigman et al. (1973) and Zigman and Vaughan (1974) demonstrated that photo-oxidation of free tryptophan yielded pigmented and fluorescent species which would bind to lens crystallins in vitro. This raised the possibility that the target of photo-oxidation could be either free or protein-bound tryptophan. Numerous investigators turned to the study of brunescent nuclear cataracts since those lenses have the greatest concentration of the pigment and of the non-tryptophan fluorescence. The search for clear decreases in the levels of tryptophan in the proteins of such lenses or in their free amino acid pool has not been successful to date (Dilley and Pirie 1974; Pirie and Dilley 1974; Zigler et al. 1976). It should be noted however that tryptophan compromises less than 2% of total amino acid in crystallins and, thus small changes would be difficult to detect especially in view of the inherent problems of tryptophan analysis.
Lerman (1980b) suggests that in the normal lens less than 20% of the protein tryptophan is susceptible to photo-oxidative damage. Studies on the novel blue fluorescence of aging crystallins, particularly the insoluble fraction from brunescent lens nuclei suggest the presence of a number of related species (Lerman 1980b). The species with emissions in the visible are generally more concentrated in nuclear cataracts. There is some evidence that this fluorescence may be concentrated in certain crystalline polypeptides (Zigman 1981). It has also been demonstrated that the formation of non-disulfide covalent crosslinks between crystallin polypeptides is associated with the heavily pigmented protein fraction and can be generated in vitro by irradiation (Buckingham and Pirie 1972). Additional photoproducts of tryptophan, including B-carbolines (Dillon, Spector and Nakanishi 1976) and anthranilic acid (Truscott, Faull, and Augusteyn 1977) have been identified from the crystallins of nuclear cataracts. Dillon and Spector (1980), Dillon et al. (1982) and Borkman, Tassin, and Lerman (1981) have studied the photolysis of free tryptophan, or tryptophan containing peptides, and of isolated lens crystallins. Analysis of these data suggests that a variety of products are possible and that the microenvironment of individual tryptophan residues is of paramount importance. Additionally, photolysis is much faster in the presence of oxygen.
Harding and Dilley (1976), however, raised two objections to the idea that sunlight caused brown nuclear cataracts. First, they pointed out that the lens damage is in the nucleus whereas the shortest wavelengths reaching the lens; i.e., those which might be absorbed by tryptophan are probably absorbed in the anterior lens cortex. Indeed as noted above cataracts induced in animals by UV-B are located in the anterior cortex. Secondly, the lack of evidence for loss of tryptophan in brown cataracts was cited. While these arguments are difficult to rebut in terms of mechanisms in which UV oxidation of tryptophan is the central event, there is another mechanism of photo-oxidation for which these objections may be less significant.
Recently, there has been increasing interest in the possible role of photosensitized processes, particularly with the involvement of activated species of oxygen, in the oxidative damage observed in the human lens. This work was spurred by data demonstrating light-mediated lens damage with such photosensitizing drugs as 8-methoxypsoralen, phenothiazines, and tetracycline and by the studies cited above by Pirie and Van Heyningen showing accelerated UV effects on crystallins in the presence of kynurenines and related compounds. Zigler and Goosey (1981) have demonstrated that several of these compounds endogenous to human lenses are capable of generating singlet molecular oxygen, a highly reactive species capable of damaging proteins as well as other biological molecules and structures. The ability of this oxidant to induce the oxidative changes characteristic of human crystallins has been established in vitro. It has also been demonstrated that such photosensitizing activity is present in the heavily altered insoluble lens crystallins from brunescent cataracts and to a lesser extent in the soluble crystallins of normal human lenses as well. Based on experiments in vitro, such a photosensitized process could account for the generation of each of the oxidative modifications presently known to occur in lens crystallins. Furthermore such a process seems consistent with nuclear localization of damage. In the lens nucleus there is no repair or replacement of altered molecules, thus allowing progressive accumulation of crystallins with oxidized residues some of which are photodynamic sensitizers. The UV-A which these species absorb will readily penetrate to the nucleus. Additionally while the lens cortex is protected by a battery of antioxidant defenses it is known that these defenses are markedly reduced in the nucleus (Hata and Hockwin 1977; Fecondo and Augusteyn 1983).
One could envision a system in which there was a slow rate of photo-oxidation ongoing in the lens controlled by antioxidant defenses and by the greatly reduced oxygen tension in the lens nucleus. The oxidative stress might have several components including direct UV oxidation of tryptophan (primarily UV-B), the high levels of H2O2 in the aqueous humor (Spector and Gardner 1982) and photosensitized oxidation involving activated species of oxygen. It has been recently demonstrated that even fetal human lenses contain low molecular weight chromophores which can generate singlet oxygen when irradiated with UV-A. The initial photochemical event could be absorption by such chromophores or direct photo-oxidation of tryptophan to produce N-formyl kynurenine (NFK) a known photodynamic sensitizer. In either case, it seems likely that the continued build-up of oxidized products in the nucleus is likely due primarily to a sensitized process, since such a process would not require large-scale tryptophan loss nor would it require penetration of UV-B into the nucleus. A relatively small number of stable sensitizing species such as NFK, bound within the long-lived nuclear crystallins, could continue to generate singlet oxygen indefinitely with the gradual accumulation of the various oxidative protein change s outlined above cumulating in aggregation and insolubilization of much of the nuclear crystallins in advanced brunescent cataracts.
Several suggestions have been made that attempt to reconcile the finding that tryptophan is not reduced in senile cataracts (as compared to normal lenses) with the hypothesis that it acts as an important endogenous photosensitizer. For example, it has been suggested that tryptophan is oxidized to a reactive molecule that simply transfers its energy to some other cell component from the excited state. It then returns to the ground state without being destroyed in the process, yet photosensitizes damage to other lens macromolecules (Weiter and Finch 1975; Weiter and Subramanian 1978). Another explanation is that free tryptophan rather than protein bound tryptophan may be destroyed when it acts as a photosensitizer, whereas tryptophan incorporated within lens proteins is unaffected. The level of free tryptophan may be reduced in cataract (Zigler et al. 1976). The failure of protein-incorporated tryptophans to be photo-oxidized might be explained by the fact that they lie in very different micro-environments that render them less susceptible to photo-oxidation (Lerman, 1980b). A third suggestion to explain the finding that tryptophan is not decreased in cataractous lenses is that only a small percent of the protein-incorporated tryptophan may be susceptible to photo-oxidation and small losses in these susceptible tryptophan might not be detectable with current analytical capabilities. Tryptophan and its photoproducts have become the most frequent culprits implicated in causing photo-oxidative changes in lens proteins, changes that are hypothesized to be responsible for senile cataractogenesis.
Photo-oxidation of lens proteins has become a major topic in lens research and has been shown to be induced by photosensitizers that are either endogenous (Zigler and Goosey 1981) or externally applied (Goosey, Zigler, and Kinoshita 1980) to the lens. One effect frequently found to occur as a direct effect of UV or as an effect of UV plus a photosensitizer is crosslinking of lens proteins (Buckingham and Pirie 1972; Goosey, Zigler, and Kinoshita 1980).
Interest in agents that can cross-link or otherwise aggregate lens proteins has been high since Benedek (1971) first proposed that the mechanisms underlying senile cataract formation was aggregation and insolubilization of lens crystallins. The suggested that aggregated lens proteins would serve as light scattering particles in the lens. The search was then underway for these high molecular weight aggregates, but unfortunately, the research produced very inconsistent and conflicting results. Some research groups found an increase in the amount of high molecular weight proteins in senile cataractous lenses while other groups found no difference in the amount of high molecular weight proteins in cataractous lenses compared to that present in lenses in age matched controls (Harding and Dilley 1976). It was also suggested that aggregation was an artifact resulting from the extraction procedure (Harding 1972). Analogous studies have more recently been carried out on lenses in which the cataracts were separated according to the nature of the changes present in the lens. The results of those studies have suggested that nuclear brunescent, but not cortical cataract, is associated with formation of increasing amounts of water insoluble proteins (Truscott and Augusteyn 1977). Some researchers have attributed this increase to cross-linking of lens proteins induced by photo-oxidation. It has been further suggested that the reason these changes occur more readily in the lens nucleus than in the cortex is that the cortex has a higher concentration of protective anti-oxidants.
If Benedek's (1971) original hypothesis that the mechanism underlying senile cataract formation was aggregation and insolubilization of lens crystallins is correct, then one would expect to find an increase in protein cross-linking and aggregation in lenses that show light scattering. To the contrary, lenses with light scattering opacities in the cortex do not show an increase in high molecular weight proteins or crosslinks (Truscott and Augusteyn 1977; Anderson, Wright, and Spector 1979). Instead, reports in the literature suggest that there may be an increase in protein aggregates in lenses with substantial nuclear brunescence. This is rather a surprise because nuclear brunescence per se is not a light scattering change in the lens; rather, the lens turns a yellow or a brown color that absorbs rather than scatters light, reducing its intensity on the retina (Lerman and Borkman 1976). The effect of brunescence is, therefore, analogous to placing a transparent filter before the eye.
Unfortunately, only man and a very few other diurnal species have been found to develop lens browning so that it has been difficult to find an animal model in which to test the hypothesis that browning can be induced in vivo by exposure to UV radiation. Therefore, those studies that have examined the effects of chronic UV exposure in experimental animals have not demonstrated that UV induces lens browning. They have, however, consistently demonstrated that UV exposure induces light-scattering cortical opacities (Bachem 1956; Zigman and Vaughan 1974; Pitts, Hacker, and Parr 1977). Therefore, though we may lack a suitable model for nuclear brunescence, there are animal models that can be used to study the induction of cortical opacities by UV radiation. These models mimic the common and considerably more visually disturbing senile cortical cataracts in man.
In those studies on UV-induced lens changes in which histology of the lenses were examined, epithelial cell changes were a uniform finding (Zigman and Vaughan 1974; Pitts, Hacker, and Parr 1977). In their study of chronically exposed mice, Zigman and Vaughan (1974) noted the similarity of the lens changes to changes induced by X-irradiation. For example, lens cells appeared to have lost their capacity to differentiate and were found to have migrated to the posterior pole, a situation that has also been found to be associated with senile cortical cataracts (Streeten and Eshaghian 1978).
While biochemical approaches have generally concentrated on nuclear cataracts with respect to UV-mediated effects, there has been recent interest in photosensitized reactions in the aqueous humor as sources of damage to lens membranes and, hence, as a possible initiator of cortical (osmotic) cataracts.
Varma, Kumar, and Richards (1979) have been investigating the possibility that a photochemical conversion of molecular oxygen present in the aqueous humor and lens into superoxide and subsequent derivatization of superoxide to other potent oxidants such as hydrogen peroxide, hydroxyl radical and singlet oxygen may be involved in initiating a cascade of toxic biochemical reactions leading to the formation of cataracts. Thus, according to this hypothesis, cataractogenic influence of light is mediated by a photochemical generation of superoxide from the ambient oxygen. Spin restriction offered by the molecular oxygen makes the formation of superoxide a necessary event in most oxidation reactions involving oxygen. It is commonly understood that, like many other free radicals, superoxide and its derivatives, if allowed to remain unscavenged for any length of time in a biological milieu, will initiate many nonspecific and deleterious reactions such as an upsetting of the normal redox chain, oxidation of vitally important protein and nonprotein - SH, peroxidation of membrane and cytosolar lipids, and polymerization and depolymerization of macromolecules such as proteins and hyaluronic acids. The cataractogenic influence of these oxidants is likely to be modulated by certain endogenous protective mechanisms. Superoxide dismutase (SOD), catalase and perioxidase constitute the first line of defense against the toxic effects of those oxygen species. Jernigan et al. (1981) showed that singlet oxygen generated in the medium has similar effects. Varma, Beachy, and Richards (1982) have demonstrated lipid peroxidation in cultured lenses irradiated with fluorescent light. While such studies are of great interest in terms of the effects of these oxidants on the lens, at the present time there is no real evidence that they are produced insignificant quantities in the ocular humors by light mediated process.
Considerable effort has been directed at elucidating mechanisms by which UV radiation (alone or in combination with exogenous photosensitizers) might induce cataract formation. As previously mentioned, it has been shown that UV radiation can induce cross-linking and aggregation of proteins. As has also been mentioned, protein aggregation may be associated with nuclear changes but it is not a characteristic associated with cortical cataracts. In may therefore be more relevant to consider the possibility that UV damage to lens proteins induces local perturbations in macromolecular structure and function. This might in turn result in localized changes in lens structure of function. For example, transport systems and ATPase (Varma, Kumar, and Richards 1979) in the lens have been shown to be inhibited by UV radiation. If the lens transport systems are damaged by UV radiation, the osmotic balance will be disrupted which, in turn, would produce major changes in lens morphology.
The UV effects on transport systems may occur due to direct UV absorption by and damage to the related enzymes; however, other mechanisms may also contributed to this inhibition. for example, the activity of transport enzymes could be dramatically altered secondary to UV induced disruption of lipids in lens membranes; and, lipid peroxidation has been proposed as a mechanisms of cataract formation (Goosey, Allison, and Garcia 1983). The resulting alterations in lipid structure could of themselves make the membranes leaky or, as just mentioned, such alterations could disrupt the structure and function of membrane proteins. If one can extrapolate from the large amount of research that has been done on other cell systems, then it may be predicted that fairly long wavelengths of UV will similarly be able to produce membrane damaging effects in the lens (Moss and Smith 1981; Imbrie and Murphy 1982; Sprott, Martin, and Schneider 1976).
If we are to determine the role of lipid peroxidation or inactivation of specific enzyme systems in the development of UV cataracts, it is critically important that lenses be sampled several intervals prior to the onset and during the development of lens opacities. Once the lens is fully opacified, retrospectively, it is difficult to state with any assurance that some observed biochemical change was the specific change that initiated the cataract.
For example, if an ATPase were found to be inactivated after a particular UV-induced cataract was fully developed, one might conclude that this inactivation had caused cell swelling, membrane lysis, and an "osmotic cataract." But it is also possible that the primary cause of the cataract was direct membrane damage or inhibition of protein synthesis and, that, only secondarily was ATPase activity involved. Therefore, to show that ATPase inactivation is of any real significance in the etiology of the cataract, it is necessary to demonstrate that ATPase inactivation precedes development of the lens opacities. In the case of UV-induced cataracts, one also needs to demonstrate that those wavelengths that inactivate the ATPase are those same wavelengths that most readily induce cataract formation.
It may be misleading to examine effects of UV (with or without added photosensitizers) on lens macromolecules isolated in test tubes because UV effects in vitro may be very different from the effects of UV on the intact lens. For example, it is possible to cause tryptophan destruction in lens proteins irradiated at a concentration of 2.5 mg/ml in a test tube, but the same type of irradiation was not found to produce such effects at the concentration of protein in the lens (Dillon et al. 1982). Also, the action spectrum may differ considerably from the absorption spectrum (Turro and Lamola 1977). The action spectrum shifts markedly in situ in the case of the photosensitizer, psoralen. Psoralen, riboflavin, methylene blue and rose bengal have all been found to induce photo-oxidative changes in isolated lens proteins when these proteins are irradiated with UV. However, each of these photosensitizers requires the presence of oxygen, otherwise lens proteins are not damaged. The amount of oxygen in the lens is not high (Kwan, Ninikoski, and Hunt 1972; Barr and Roetman 1974). Thus, these findings must be extrapolated to the in vivo lens with considerable discretion and it remains to be determined just how much photodynamic damage (that is damage where oxygen is involved as an intermediate) can be produced in the lens in situ. This is a very important research question because any effect that is of significance as a mechanisms of cataract formation must be inducible in a lens that is still in the eye. For example, it might be interesting to use 254 nm radiation as a toll in vitro to demonstrate that the lens epithelium has the capacity to repair damaged DNA (Jose and Yielding 1977). However, 254 nm radiation does not penetrate the cornea, so it will not be hazard for the in vivo lens and it will not cause cataracts. Therefore, it is a far more significant finding that 300 nm radiation can induce DNA repair synthesis in the lens epithelium when the lens is exposed in situ through the intact cornea (Jose, Kock, and Respondek 1982; Brenner and Grabner 1982). This finding shows that 300nm UV radiation penetrates through the cornea and demonstrates that epithelial cell DNA is a target of its effects.
Therefore, if we are to establish mechanisms of UV damage in the lens, we must be able to show that a given damaging reaction can be produced by UV in the in situ lens. An effect that can be produced on lens macromolecules isolated in a test tube may be interesting and stimulate further research, but it must not be taken as sufficient proof that such an effect can occur in vitro. This is especially true for those reactions that require oxygen.
One photosensitizer that may provide important clues as to mechanisms involved in cataract formation is the drug psoralen which in combination with UV radiation is used in treating psoriasis. Two different mechanisms have been proposed to underlie the psoralen cataract. According to one hypothesis, the cataracts occur as a consequence of psoralen binding covalently to lens proteins which results in formation of additional damaging photosensitizers in the lens (Lerman, Megaw, and Willis 1980). The second hypothesis is that the cataracts occur as a consequence of psoralen induced damage to lens nucleic acids (Jose and Yielding 1979).
It has been found that intense exposure to UV radiation will cause psoralen to bind to isolated lens proteins, specifically the tryptophan moieties (Lerman, Megaw, and Willis 1980); however, the reaction requires oxygen (Megaw, Lee and Lerman 1980). Therefore, it is important to determine whether such protein binding occurs in the intact lens. Although spectra have been presented that demonstrate that UV radiation induces psoralen binding in the in vivo lens, it is not clear that the binding observed represents protein specific binding or whether the binding is to some other lens macromolecule. Lens protein fractions have been extracted from psoralen-treated humans or laboratory animals and fluorescence spectra taken from these. These spectra were interpreted to demonstrate photobinding of psoralen to lens proteins; however, no effort was made in these studies to extract nucleic acids from these "protein" preparations. It is very possible that a significant amount of the binding that was observed is accounted for by psoralen binding to RNA or DNA present in the degenerating nuclei of the lens cortex. The possibility that the spectra represented binding to nucleic acids in the differentiating fibers was discounted by the researchers because there is such an overwhelming concentration of protein compared to DAN in lens fibers (Lerman, Megaw, and Gardner 1982a). This is a reasonable assumption if a significant percentage of the psoralen were actually bound to the protein. However, the argument would be nullified if the majority of the psoralen were found to be bound to the small amount of nucleic acids in the fibers. Autoradiograms showing psoralen binding in the lens suggest that the latter is in fact the case (Lerman et al, 1981). Examination of those autoradiograms shows pronounced binding of psoralen in the epithelium plus discrete binding to the degenerating fiber nuclei. In comparison, binding to any other portions of the fibers is not distinguishable from background grains.
Autoradiography has also demonstrated psoralen binding in the nucleated lawyers of the retina and cornea but no binding to the non-nucleated layers of these tissues (Lerman et al, 1981). It is rather difficult to reconcile the failure of psoralen to bind to the non-nucleated regions of the retina and cornea with the suggestion that it binds significantly to cortical fiber proteins. A trivial explanation for this difference is that lens proteins are somehow "different" from corneal and retinal proteins, making the former susceptible to psoralen binding and the later not. This argument is rather difficult to reconcile with the requirement of oxygen for photo-induction of psoralen linking to proteins, a requirement that would lead one to expect greater binding to the proteins in the highly oxygenated retina and cornea compared to the relatively anoxic lens.
If lens proteins are the major target of psoralen's action on the lens, then one would expect that the cortical fibers would be disrupted as a primary event. In fact, however, histologic observations have shown that the first target of psoralen's effects are the epithelial cell (Jose, Kock, and Respondek 1982). Only after very considerable damage is observable in the epithelium is any damage detectable in the lens cortex which is consistent with DNA as a primary target of psoralen damage. That psoralen plus UVB can induce DNA damage in the lens is shown be the finding that the combination induces DNA repair in lens epithelial cells (Jose and Yielding 1979). It will be interesting to resolve the question as to the relative roles of specific macromolecular targets in development of psoralen-induced cataracts.
As with psoralen, most other studies of UV effects on the lens have directed their major emphasis toward examinations of changes in lens proteins. But, as with the psoralen study, there are compelling reasons to consider other macromolecules as targets of UV damage. Lens lipids have been mentioned previously and interest in lipids peroxidizing effects is of current interest in many laboratories. However, interest in the effect of UV and photosensitizers on lens DNA is currently very limited. The role of DNA damage in development of lens cortical opacities in generally overlooked or rationalized away. Some have assumed that there is so little DNA in the lens that even if it were a target, any effects on it would be overwhelmed by effects on lens proteins. There are, however, good reasons to consider the lens to be similar to other biological systems in which DNA is a significant target of UV damage.
If one considers the action spectrum that has been determined in those studies in which specific wavelengths of UV were isolated to examine their cataractogenic potential, it is noted that these lie in the range of 290 to 320 nm (Bachem 1956; Pitts, Hacker, and Parr 1977). these are the same wavelengths that most readily produce thymidine dimers in skin (Pathak, Kramer, and Guengerich 1972). Shorter wavelengths are blocked from deeper layers of the skin by the stratum corneum, quite analogous to corneal UV absorption which protects the lens. These are the same wavelengths which most readily induce malignant transformation and skin cancer (Freeman 1975). UV radiation up to 320 nm also can induce DNA repair synthesis in cultured fibroblasts (Ichihashi and Ramsey, 1976). Longer wavelengths of UV do not induce skin cancer, although they may, if applied very intensely, induce strand breaks and other damage in DNA (Webb and Peak 1981, Harm 1978). Endogenous photosensitizers are likely to be involved since DNA itself does not absorb appreciably at that wavelength. Long wave UV may potentate the action of short wave UV in cancer induction and it can also be noted that 354 nm has been found to inactivate DNA repair mechanisms (Tyrell 1976).
With all the interest in tryptophan as an endogenous photosensitizer of protein damage in the lens, it is interesting to point out parenthetically that photo-oxidation of tryptophan has also been found to induce DNA damage in lower organisms as a consequence of production of H2O2 (Ananthaswamy and Eisenstark, 1976). The possibility that those free radical species that have been found to be generated from UV excitation of tryptophan in the lens may also act on lens DNA as a target should not be overlooked. Furthermore, tryptophan photoproducts have also been found to bind to DNA (Glazer, Rincon, and Eisenstark 1976) and to inhibit strand rejoining in damaged DNA (Yoakum et al. 1974).
Another reason to consider that DNA is a target of UV damage in the lens is the finding that 300 nm radiation can induce DNA repair synthesis in the lens epithelium, even when the irradiation is applied through the intact cornea (Jose, Kock, and Respondek 1982; Brenner and Grabner 1982) and DNA repair synthesis is an indication that DNA was damaged. This point is often misunderstood, repair is not a perfect process and it should not be assumed that nay time that DNA is damaged and repair is undertaken; thus, anything that can induce DNA repair must be recognized for its primary damaging effects. Errors induced in the genome of lens cells may manifest themselves as mutational events in the target epithelial cell. Such mutational events would be cumulative over an individual's lifetime. Those cells that have undergone mutations might loose their capacity to differentiate into normal lens fibers and, for example, pile up at the posterior pole. The later is seen in the senile cataract (Streeten and Eshaghian 1978) as well as in animals exposed to near UV radiation (Zigman and Vaughan 1974). The cells may also not carry out their normal functions such as maintaining less osmolarity. Then we might develop what appeared to be an "osmotic" cataract, when in fact, the underlying mechanism was genetic.
Epidemiological studies have identified a correlation between the prevalence of various types of cataracts in humans and the flux of sunlight or ultraviolet radiation reaching the earth's surface (Hiller, Giacometti and Yuen 1977, Zigman, Datiler, and Torczynski 1979; Taylor 1980, Hollows and Moran 1981). Hiller, Sperduto and Ederer (1983) developed a multivariate logistic risk function that describes the correlation found between the prevalence of senile cataracts and the flux of UV-B and other risk factors. The results of this study may indicate the magnitude of change in the prevalence of senile cataracts that could be associated with changes in UV-B flux due to ozone depletion.
Hiller, Sperduto and Ederer based their analysis on the 1971-1972 National Health and Nutrition Examination Survey (HANES) general medical and ophthalmological examinations of over 10,000 persons, ages 1-74 years. Using the HANES data, persons were assigned to one of two mutually exclusive groups: (1) cataract or aphakia (either eye), and (2) neither cataract nor aphakia (either eye). Cataract was defined as "senile lens changes (cortical, nuclear, posterior-subcapsular, or other) consistent with best corrected visual acuity of 20/30 (6/9) or worse" (Hiller, Sperduto and Ederer (1983) p. 240). This definition differs from the HANES survey, which used a visual acuity of 20/25 (6/7.5) or worse. Aphakia was diagnosed when "the lens had been surgically removed and there was no history of congenital, traumatic or secondary cataract" (Hiller, Sperduto and Ederer (1983) p. 240). The study by Hiller, Sperduto and Ederer included HANES data on a total of 2,225 persons between the ages of 45 and 74 years who had resided at least one half of their life in the state where the HANES examination took place. Of these 2,225 people, 413 (18.6 percent) were placed in the cataract or aphakia outcome category.
The UV-B data were developed by NOAA for the 35 HANES locations used in the study based on a statistical analysis of UV-B data collected at 10 locations using Robertson-Berger meters (RB-meters). The statistical analysis incorporates season, latitude, elevation, weather (clouds), and haze. Subsequent validation of the estimates at six locations indicated that the differences between the estimated and observed mean daily flux average about seven percent.
These data on UV-B and outcome (i.e., cataract) were used in conjunction with demographic and medical history data to estimate the following multivariate logistic risk function:
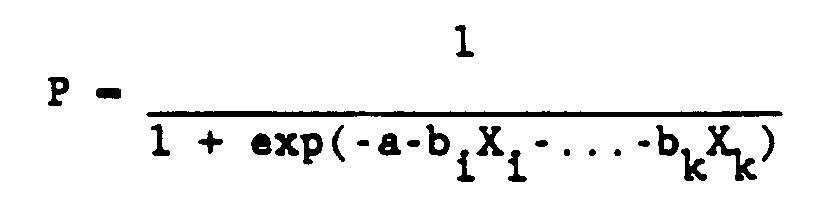
where P is the probability (or risk) of having a cataract, and Xi are risk factors. In addition to UV-B, the following risk factors were analyzed: age; race; sex; education; diabetes; systolic blood pressure; and residence (urban, rural).
Exhibit 10-12 displays the standardized regression coefficients estimated for each of the risk factors. Positive coefficients indicate factors that are correlated with increased risk, negative coefficients indicate factors that are correlated with decreased risk. The coefficients presented in the exhibit are "standardized," meaning that they represent the expected change in the logit of P (equal to ln (P/1-P)) for a one standard deviation change in the risk factor. Standardization of the coefficients allows the relative importance of the risk factors to be identified by the relative size of the standardized coefficients.
As shown in Exhibit 10-12, three risk functions were estimated: (1) univariate (outcomes as a function of the risk factor); (2) bivariate (outcome as a function of the risk factor and age); and (3) outcome as a function of all the risk factors simultaneously. For all three formulations, UV-B is statistically significant, and positively correlated with the increased risk of being in the cataract outcome category.
Using the multivariate risk function coefficients, and the mean values for all the risk factors other than UV-B, the change in the prevalence of cataract for each 1.0 percent change in UV-B is estimated to be approximately 0.5 percent. This relationship holds for changes in UV-B as large as minus 20 percent to plus 30 percent. Outside of this range, reductions in UV-B are associated with less of a reduction in cataract prevalence, and increases in UV-B are associated with larger increases.
Of note is that this estimated relationship between UV-B and cataract prevalence varies with age; UV-B has a larger effect on prevalence (on a percentage basis) among younger individuals. Exhibit 10-13 displays the percent increase in cataract prevalence expected due to increases in UV-B, for peoples of different ages. As shown in the exhibit, the percentage increase in prevalence due to changes in UV-B are estimated to be larger for 50 year olds than for 70 year olds.
Although the effect of UV-B on prevalence is estimated to be larger at younger ages (on a percentage basis) using the multivariate risk function, the prevalence of senile cataracts is known to increase substantially with age. Leske and Sperduto (1983) report the prevalence of senile cataracts in both sexes found in the Framingham Eye Study to be as follows: 52 to 64 years old -- 4.5 percent; 65 to 74 years old -- 18.0 percent; 75 to 85 years old -- 45.9 percent. These prevalence estimates use the same definition of cataracts as used by Hiller, Sperduto, and Ederer. (Larger prevalence rates are reported by Leske and Sperduto based on HANES data. These estimates, however, use a definition of cataract that includes a decrease in vision to 20/25 (6/7.5), instead of the 20/30 (6/9) used in both the Framingham study and the Hiller, Sperduto, and Ederer risk study.) Because cataracts are more prevalent in older individuals, increases in the actual number of cases of cataracts would likely be larger for older individuals, even though the percentage increase in risk has been estimated to be larger for younger individuals.
Using the prevalence data cited above, the prevalence of cataracts in the U.S. population is on the order of 9.3 million. Using the multivariate risk function (with all values set to their means except age and UV-B) the hypothetical increased prevalence for 1985 that would have occurred had the entire population experienced 1.0 percent ozone depletion can be estimated. For a 1.0 percent depletion, the annual UV-B flux measured on the RB-meter has been estimated to increase by approximately 0.83 percent (see Serafino and Frederick (in press) and Chapter 17 for a discussion of the relationship between ozone depletion and UV flux). A 0.83 percent increase in UV-B is associated with increases in cataract prevalence that varies by age. Across all the ages, prevalence would be expected to be about 0.26 percent higher, or about 24,000 cases, had ozone been depleted by 1.0 percent.
Of note is that the RB-meter measure of UV radiation may not be the appropriate action spectrum to use to evaluate the potential biological effects of increased UV-B such as cataract. For example, the DNA action spectrum may be preferred. Even though the RB-meter and DNA action spectra are highly correlated in the range of current observations, because the DNA action spectrum is more heavily weighted toward shorter wavelengths, it increases more rapidly with decreases in ozone levels; a 1.0 percent depletion would lead to approximately a 2.0 percent increase in UV-B. Using the 2.0 percent increase in UV-B would yield about a 0.6 percent increase in prevalence, or about 57,000 cases.
There are various important limitations in the use of these estimates and data. The correlation between UV-B and cataracts reported by Hiller, Sperduto and Ederer does not prove a causal connection -- other (unknown) factors could be playing a role. These (unknown) factors would have to be correlated with UV-B flux. Also, the study does not have estimates of individual lifetime UV-B exposure, thereby limiting the strength of the evidence for the association between UV-B exposure and cataracts. Additionally, the sample population analyzed may not be representative of the entire U.S. population. Finally, the outcome category used in the study does not differentiate between different types of cataracts, some of which may be more strongly related to UV-B exposure than others.
Confidence in the estimates developed here are strengthened by several considerations. The correlation between WV-B flux and sunlight flux is high, and a correlation between sunlight and cataracts has also been found in Australia (Taylor 1980) and in China (Mao and Hu 1982). An association between UV-B exposure and cataract has also been demonstrated in laboratory animals. Therefore, although considerable investigation remains to be performed, indications are that the association between UV-B and cataract is a reasonable basis for evaluating potential impacts due to increased UV-B flux associated with ozone depletion.
Stable Retinal Disorders
Evidence has been adduced which provides a chain of evidence on which to postulate that optical radiation may contribute to human retinal disorders which are not progressive, that is, stable retinal problems. This chain starts with the knowledge that UV-B, UV-A and light are absorbed by various retinal tissues (Wolbarsht 1976; Warner 1982). Moreover, the amount of radiant energy incident on the retina is dependent on the transparency of the cornea and lens, and on pupil diameter. Next a large number of bioaffects of these wavelengths have been identified which are hazardous to the retina (Marshal 1970; Williams and Baker 1980). Further, empirical estimates have been made of the domain of time, intensity, and wavelength which can induce damage to the pigment epithelium, the photoreceptors and the inner retina. These estimates provide sufficient quantitative evidence to establish safety standards to protect people from the short-term retinal damage which could be induced by lasers and other intense sources of optical radiation (Sliney and Wolbarsht 1980; Pitts 1973). There is, moreover, sufficient evidence of additive and persistent changes in the monkey and human retina to hypothesize that some stable long-term visual impairments in humans may be related to optical radiation. There are reports that optical radiation producers retinal damage hours, days, months and years after irritation. Although sparse, this evidence is instructive. For example, sungazing by humans has been reported to produce persistent, evolving and delayed alterations in the visual system. Cavonius, Elgin, and Robbins (1974) reported that aniseikonia may persist for years. Pigmentary patches and blind spots have been reported to evolve in a complicated fashion over many months after solar retinopathy (Lowenstein and Steel 1941). In addition, late complications resulting in reduced visual acuity have been described (McFaul 1969). Furthermore there are several reports in humans of prolonged, and even cumulative, impairments in dark adaptation following extended viewing by humans of skylight (Hecht et al. 1948; Clark, Johnson, and Dreher 1946) or bright blue, violet (Brindley 1953) and ultraviolet (Wolfe 1949) radiation. Also there is at least one report of persistent visual impairment resulting from the accidental exposure to ultraviolet radiation from a welding arc (Naidoff and Sliney 1974). Even more important is the description by Tso and Woodford (1983) of sub-RPE neovascularization and late impairment in fluid transport after several years of evolving changes in regional pathology following excessive exposure to optical radiation.
Some of these effects are the result of injury to the retina which was opthalmoscopically visible, some of these effects resulted from exposure to optical radiation which did not produce opthalmoscopically visible damage. Most important are two demonstrations that the addition of subthreshold exposures separated by several days induces retinal damage (Greiss and Blankenstein 1981; Kuwabara and Okisake 1976). The data strongly suggest that retinal damage induced by optical radiation can cumulate over days. This is especially important since the concern about long-term visual health problems necessarily involves consideration of the intermittent nature of exposure to emissions from multiple sources over an extended period of time.
Retinal Degeneration
There are some reasons to suspect that retinal damage induced by ultraviolet radiation can be unstable, that is, produce progressive damage. The retina of monkeys can be damaged by ultraviolet radiation (Ham 1984; Sykes et al. 1981). This damage to the retinal pigment epithelium, to the photoreceptors and the neurons is dose dependent, photochemically mediated and cumulative over hours and days. Although very few long-term observations have been made on the primate retina following excessive exposure to optical radiation, there are theoretical reasons to suspect that a retina compromised by disease or age would be more vulnerable to any degenerative changes which could be induced by optical radiation (Young 1981). The combination of short-wavelength optical radiation, oxygen, chromophores, and photosensitizers in the retina is potent with possibilities for producing retinal degeneration, although the only direct evidence of the exacerbation of retinal degeneration by optical radiation has been obtained in rodents (LaVail and Batelle 1975). In addition, the higher retinal doses of UV received by aphakes and pseudophakes make these individuals especially vulnerable to systoid macular edema (Kraff et al. 1985). Fluorescein leakage occurs following exposure of the retina of the aphakic monkey eye to UV-A radiation, but comparable studies using UV-B radiation have not been conducted. Therefore, retinal degeneration should be considered a risk of excessive exposure to ultraviolet radiation.
Very little research has been conducted on primates, on diurnal animal models of human retinal degenerations, or on retinas compromised by chemicals, age or other agents. The striking evidence of long-delayed and persistent changes in the monkey retina following optical radiation, coupled with some clinical evidence in humans, suggests that research on primates needs emphasis.
Aging Disorders
Deterioration of the visual system as individuals age is a fact (Owsley et al. 1986). The likelihood that optical radiation contributes to this deterioration is high for several reasons. First, the cellular pathology observed in older individuals is similar in appearance to radiation induced damage (Kuwabara 1978). Second, the opthalmoscopic appearance of the aged retina is sometimes similar to that induced by optical radiation (Klein 1958). Third, some of the kinds of vision loss described in the aged are consistent with the losses which could be produced by optical radiation damage (Jaffe, de Monestario, and Podgor 1982; Harweth and Sperling 1975). Fourth, the accumulation of lipofuscin materials in the RPE and other changes in the retina suggest that the aged retina has a lessened capability of repair (Feeney-Burns, Berman, and Rothman 1980).
Optical radiation may have quite different visual health consequences for eyes with and without ocular disease. Optical radiation may accelerate the deterioration of central vision in older individuals with central retinal disease (Hyman et al. 1983), whereas in those free of ocular disease optical radiation may impair paramacular and peripheral vision more than foveal vision. This interpretation is offered to reconcile the facts of visual loss outside the macular in disease-free aged eyes (Owsley et al. 1986) with Young's prediction that the central retina will exhibit the major effects of lifetime retinal exposure to optical radiation (Young 1981). This ad hoc explanation is not very satisfactory but it does indicate the need to temporize theory with data. Other interpretations also are possible, e.g., those individuals with central retinal disease may have had a different exposure history or some genetic vulnerability.
Given these facts and theoretical considerations, exposure of the aged retina to ultraviolet radiation in excess of amounts permitted by the phakic eye probably is hazardous. The retina of the aged individual is exposed to more radiation than phakics receive when the crystalline lens is extracted without placing a comparable filter in front of the retina, even when ordinary sunglasses are used.
Research is needed to determine the extent to which optical radiation is a factor in the deterioration of the vision of the elderly. In the meantime, we should take practical steps to protect our eyes from excessive optical radiation, and thus prolong our visual lifetime.
Development Disorders
While there are several reasons to suspect hazardous effects of optical radiation on visual development, almost no research has been conducted on the developing primate retina. In fact, there is very little experimental exposure data on the developing retina of any species. Glass et al. (1985) have reported that the probability of retinopathy of prematurity (ROP) is highest in infants exposed to more light during their hospital stay. This evidence confirms a prediction by Wolhbarsht et al. (1983) about ROP and light and is consistent with the evidence for the interaction of oxygen and light in retinal damage (Ruffolo et al. 1984). The action spectrum for this effect in unknown but UV-B and UV-A probably play a role. Furthermore, there are large variety of sources and situations in which infants are exposed to optical radiation. Some of the same evidence which gave rise to suspicions about the role of optical radiation in retinal degeneration and visual aging is relevant to the potential hazards to visual development: short-wavelength radiant energy incident on the retina, short-wavelength chromophores in the retina, dose-dependence of retinal damage, cumulative effects, long duration effects and delayed effects. In addition, there are special properties of the developing visual system which might decrease injury thresholds, such as more transparent ocular media and different densities of chromophores and screening pigments, or which might increase the vulnerability of infants, such as the long period of post-natal retinal development (especially foveal) and early dependence on non-foveal visual fields. Thus, the long periods required for many visual processes to mature suggests a window-of vulnerability during which time optical radiation might alter later visual performance, especially if optical radiation alters the spatial and temporal summation properties of the neural retina.
Even though there is little experimental data on the effects of optical radiation on the developing retina, the photobiological and visual science evidence is sufficient to postulate that disorders of visual development are risks of optical radiation.
Retinal Problems
The only human data from which one could derive an estimate of the radiant exposure of UV which could damage the retina is Hecht et al. (1948) and Clark, Johnson, and Dreher (1946). These data showed that staring at skylight for several hours induced an abnormal retardation of dark adaptation. Since rhodopsin has an absorption band in the UVA (Kurzel, Wolbarsht, and Yananashi 1977), this deficit could have been due to the ambient outdoor UVA. If the ambient level of UVA at the cornea was about 9 x 10[-2] Jcm[-2], as suggested by Ham and Mueller (1982), then the retinal radiant exposure might have been about 9 x 10[-3] Jcm[-2] in the Hecht and Clark studies. Experimental estimates of UV retinal damage in monkeys have been few. Schmidt and Zuclich (1980) found the threshold at 325 nm to be 10 Jcm[-2]. Recent evidence in phakic rabbits (Pitts, Bergmanson, and Chu 1983) and rats (Rapp, Jose, and Pitts 1985) suggest that the radiant exposure necessary to damage the retina is even lower at 300 nm. The threshold for 300 nm damage to the monkey retina currently is approximately 0.06 Jcm[-2]. Ham and Mueller (1982) found the aphakic monkey has thresholds of 5.0 Jcm[-2] at 325 nm, 5.4 Jcm[-2] at 350 and 8.1 Jcm[-2] at 380 nm.
1 This section in boldface type is taken from Pitts, D.G. et al. (in press)
2 This section in boldface type is taken from Waxler, M. (in press).
REFERENCES
Anathaswamy HN and A Eisenstark (1976) "Near-UV-induced breaks in phage DNA: sensitization by hydrogen peroxide (a tryptophan photoproduct)." Photochem. Photobiol. 24:439-442.
Anderson EI, DD Wright and A Spector (1979) "The state of protein sulfhydryl groups in normal and cataractous proteins II cortical and nuclear regions." Exp. Eye Res. 29: 233-243.
Augusteyn RC (1981) In Mechanisms of Cataract Formation in the Human Lens. G. Duncan (ed). Academic press.
Augusteyne R (1974) "Human lens albuminoid." Jap J. Opthalmol. 18:127-134.
Augusteyne RC (1975) "Distribution of fluorescence in the human cataractous lens." Opthalmic Res. 7:217-224.
Bachem A (1956) "Opthalmic ultraviolet action spectrum." Amer. J. Opthal. 41: 969-980.
Bando M, A Nakauima and K Satoh (1975) "Coloration of human lens protein. Exp. Eye Res. 20: 489-492.
Barker FM II (1979) The transmittance of the EMS from 200nm to 2500nm through the optical tissues of the eye of the pigmented rabbit. MS Thesis University of Houston, Houston, Texas.
Barr RE and EL Roetman (1974) "Oxygen gradients in the anterior chamber of anesthetized rabbits." Invest. Opthal. 13: 386-392.
Benedek GB (1971) "Theory of transparency of the eye." Applied Optics 10: 459-473.
Berler D and R Peyser (1982) "Light intensity and visual acuity following cataract surgery." Opthalmology 89 (Suppl.) L 117.
Bhuyan KC and DK Bhuyan (1977) "The relative functions of superoxide dismutase and catalase in the eye." IRCS Medical Science 5: 279.
Boettner EA and JR Wolter (1962) "Transmission of the ocular media." Invest. Ophth. 1(6): 766-783.
Borkman RF (1977) "Ultraviolet action spectrum for tryptophan destruction in aqueous solution." Photochem. Photobiol. 26:163-166.
Borkman RF and S Lerman (1977) "Evidence for a free radical mechanism in aging and UV irradiated ocular lenses." Ep. Eye Res. 25: 303-309.
Borkman RF, A Dalrymple and S Lerman (1977) "Ultraviolet action spectrum for fluorogen production in the ocular lens." Photochem. Photobiol. 26: 129-132.
Borkman RF, JD Tassin and S Lerman (1981) Exp. Eye. Res. 32: 747-754.
Brenner W and G Grabner (1982) "Unscheduled DNA repair in human lens epithelium following in vivo and in vitro UV irradiation." Opthal. Res. 14: 160-166.
Brilliant LB, NC Grasset, RP Pokhrel, A Kolstad, JM Lepkowski, GE Brilliant, WN Hawks and B Pararajasegaram (1983) "Associations among cataract prevalence, sunlight hours and altitude in the himalayas." Amer. J. Epidem. 118:250-264.
Brindley GC (1953) "The effects on colour vision of adaptation to very bright lights." J. Physiol. 122: 332.
Buckingham RH and A Pirie (1972). "The effect of light on lens proteins in vitro." Exp. Eye Res. 14: 297.
Castineiras SG, J Dillon and A Spector (1979) "Effects of reduction on absorption and fluorescence of human lens proteins." Exp. Eye Res. 29: 573-575.
Cavonius, CR, S Elgin, and DO Robbins (1974) "Thresholds for damage to the human retina by white light." Exp. Eye Res. 19: 543-548.
Chand D, HK El-Aguizy, RD Richards and SD Varma (1982) "Sugar cataracts in vitro: implications of oxidative stress and aldose reductase I." Exp. Eye Res. 35: 491-497.
Chand D and SD Varma (1983) "Human lens fatty acids composition." Invest. Opthal. and Vis. Sci. (ARVO suppl.) 24: 31.
Chand D, SD Varma and RD Richards (1982) "Lens lipid peroxidation. Conjugated acylpolyene and schiff bases in human cataracts." Invest. Opthal. and Vis. Sci. (ARVO suppl.) 20: 218.
Chylack LT Jr. (1978) "Classification of human cataracts." Arch Opthal. 96: 888.
Chylack LT, MR Lee, WH Tung and H-M Cheng (1983) "Classification of human senile cataractous change by the American cooperative Cataract Research Group (CCRG) Method." Invest Opthalmol. and Vis Sci, 24: 424-431.
Clark, BA, ML Johnson and RE Dreher (1946) "The effect of sunlight on dark adaptation." Am. J. Opthal. 29: 823-836.
Cooper GF and JG Robson (1969) J. Physiol. 203: 411-17.
Crylin MN, A Pedvis-Leftick and J Sugar (1980) "Cataract formation in association with ultraviolet photosensitivity." Annals of Opthalmol. 12: 786-790.
Dilley KJ and A Pirie (1974) "Changes to the proteins of the human lens nucleus in cataract." Exp. Eye Res. 19: 59-72.
Dillon J and A Spector (1980) "A comparison of aerobic and anaerobic photolysis of lens proteins." Exp. Eye Res. 31: 591-599.
Dillon J, A Spector and K Nakanishi (1976) Nature 259: 422-433.
Dillon J, M Garner, D Roy and A Spector (1982) "The photolysis of lens protein: molecular changes." Exp. Eye Res. 34: 651-658.
Doniger J, ED Jacobson, K Krell and JAD Paolo (1981) "UV light action spectra for neoplastic transformation and lethality of Syrian hamster embryo cells correlate with spectrum for pyrimidine dimer formation in cellular DNA." PNAS 78: 2378-2382.
Duke-Elder S and PA MacFaul (1972) System of Opthalmology V xiv pt 929-932.
Duke-Elder WS (1926) Lancet 1: 1188-91.
Fecondo JV and RC Augusteyn (1983) Exp. Eye Res. 36: 15-23.
Feeney-Burns L, ER Berman and H Rothman (1980) "Lipofuscin of human retinal pigment epithelium." Am. J. Ophth. 90: 783-791.
Fraunfelder F, J Megaw and S Lerman (1983) "Further studies on allopurinol in human cataracts." Invest. Opthalmol. Vis. Sci. 24 (Suppl.):3.
Freeman RG (1975) "Data on the action spectrum for UV carcinogenesis." J. Nat. Cancer Inst. 55: 1119-1121.
Garner MH and A Spector (1980) "Selective oxidation of cysteine and methionine in normal and senile cataractous lenses." Proc. Natl. Acad. Sci. 77: 1274.
Gigrus JS, S Coren and C Porac (1977) "Independence of in vivo human lens pigmentation from UV-light exposure." Vision Res. 17: 749-750.
Glass PA, G Avery, KN Sinvasubramanian, M Keys, AN Sostek and DS Friendly (1985) "Effect of bright light in the hospital nursery on the incidence of retinopathy of prematurity." N. Engl. J. Med. 313:401-
Glazer LD, A Rincon and A Eisenstark (1976) "The binding of near UV light-induced tryptophan photoproducts to DNA." Biochem. Biophys. Acta 418: 137-145.
Goosey JD, JS Zigler and JH Kinoshita (1980) "Cross-linking of lens crystallins in a photodynamic system: a process mediated by a singlet oxygen." Science 208: 1278-1280.
Goosey JD, ME Allison and CA Garcia (1983) "Lipid peroxidative mechanism for posterior subcapsular cataract formation in the rabbit." ARVO Abstracts, Invest. Opthal. Vis. Sci. 24: 75.
Greiss GA and MF Blakenstein 1981 "Additivity and repair of actinic retinal lesions." Invest. Ophth. and Vis. Sci. 20: 803-807.
Ham WT Jr (1984) "Basic mechanisms underlying the production of photochemical lesions in the mammalian retina." Curr. Eye Res. 3: 165-174.
Ham WT Jr and HA Mueller "Action spectrum for retinal injury form near ultraviolet radiation in the aphakic monkey." Am. J. Opthalmol. 93:299- .
Harding JJ (1972) "The nature and origin of the urea-insoluble protein of human lens." Exp. Eye Res. 13: 33-40.
Harding JJ (1981) In Molecular and Cellular Biology of the Eye Lens. H. Bloemendal (ed). Wiley Interscience.
Harding JJ and KJ Dilley (12976) "Structural proteins of the mammalian lens: a review with emphasis on changes in development, aging and cataract." Exp. Eye Res. 22: 1-73.
Harm, W (1978) "Evidence for photoenzymatically repairable, lethal 'non-dimer' photoproducts formed in E. coli cells by 265 nm radiation or by sunlight 360 nm." Mutat. Res. 51: 301-310.
Harweth RS and HG Sperling (1975) "Effects of intense visible radiation on the increment-threshold spectral sensitivity of the rhesus monkey eye." Vis. Res. 15: 1193-1204.
Hata N and Hockwin O (1977) Opthal. Res. 9: 256-262.
Hecht S, CD Hendley, S Ross, and PV Richmond (1984) "The effect of exposure to sunlight on night vision." Am. J. Opthal. 31. 1573-1580.
Hiller R, L Giacometti and K Yuen (1977) "Sunlight and cataract: An epidemiological investigation." Amer. J. Epidemiol. 105: 450-459.
Hiller R, RD Sperduto and F Ederer (1983) "Epidemiologic associations with cataract in the 1971-1972 national health and nutrition examination survey." 118: 239-249.
Hochemier BF (1981) "A possible cause of chronic cystyc maculopathy the operating microscope." Ann. Opthalmol. 13: 153-155.
Hockwin O, V Dragomirescu and S Lerman (1983) "In vivo age-related changes in normal and cataractous human lens density." Acta XXIV International Cong. Opthalmol. In Print.
Hoenders HL and H Bloemendal (1981) In Molecular and Cellular Biology of the Eye Lens. H. Bloemendal (ed). Wiley Intersciences.
Hollows F and D Moran (1981) "Cataract -- The Ultraviolet Risk Factor.- Lancet ii: 1249-1251.
Hymen LG, Lilienfeld, et al. (1983) "Senile macular degeneraton: case-control study" Am. J. Epidemiol. 118: 213-227.
Ichihashi M and CA Ramsey (1976) "The action spectrum and dose response studies of unscheduled DNA synthesis in normal human fibroblasts." Photochem. Photobiol. 23: 103-106.
Imbrie CW, and TM Murphy (1982) "UV action spectrum (254-405nm) for inhibition of K-stimulated and adenosine triphophosphatase from the pasma membrane of Rosa damascena." Photochem. Photobiol. 36: 537-599.
Jaffe MJ, FM de Monestario and MJ Podgor (1982) "Retinal aging and blue-sensitivity cone functioning." Invest. Ophth. Vis. Sci. 22 (Suppl.): 218.
Jernigan HM, HN Fuki, JD Goosey and JH Kinoshita (1981) "Photodynamic effects of rose bengal or riboflavin on carrier mediated transport systems in rat lens." Exp. Eye Res. 32: 461-466.
Jose JG (1978) "The role of DNA damage, its repair and its misrepair in the etiology of cataract: a review." Ophthal. Res. 10: 52-62.
Jose JG and KL Yielding (1977) "Unscheduled DNA synthesis in lens epithelium following ultraviolet irradiation." Exp. Eye Res. 24: 113-119.
Jose JG and KL Yielding (1979) "Photosensitive cataractogens, chlorpromazine and methoxypsoralen cause DNA repair synthesis in lens epithelial cells. Invest. Opthal. Vis. Sci. 17: 687-691.
Jose JG, HR Kock and A Respondek (1982) "Histological observations of the lenses of psoralem + UV-A treated albino rats and a theory as to the underlying mechanism." Graefe's Arch. Clin. Exp. Ophthalmol. 219-44-
Kahn HA and RC Milton, Revised Framingham eye study prevalence of glaucoma and diabetic retinopathy.
Kahn HA, HM Leibowitz, JP Ganley, MM Kini, T Colton, RS Wickerson and TR Dawaber (1977a) "The Framingham eye study: I. Outline and major prevalence findings." Am. J. Epidemiol. 106: 17-32.
Kahn HA, HM Leibowitz, JP Ganley, MM Kini, T Colton, RS Wickerson and TR Dawaber (1977b) The Framingham eye study: II. Association of opthalmic pathology with single variables previously measured in the Framingham Heart study." Am. J. Epidemiol. 106: 33=41.
Kalustian A, M Sun and S Zigman (1978) "N-UV light sensitized inactivation of glutathione reductase." Am. Soc. Photobiol. (Abstracts-June) 112-113.
Kinoshita J (1974) "Mechanisms of cataract formation." Invest. Ophth. Vis. Sci. 18: 713-724.
Kinsey VE (1948) "Spectral transmission of the eye to ultraviolet." Arch. Ophth. 39: 508-513.
Klien B (1958) "Diseases of the macula." Arch. Opthalmol. 60: 175-186.
Kraff MC, DR Sanders, LM Jampol and HL Lieberman (1985) "Effect of an ultraviolet-filtering intraocular lens on cystoid macular edema." Opthalmology 92:366-
Kramer SG (1980) "Epinepherine distribution after topical administration to phakic and aphakic eyes." Trans-Am Opthalmol. Soc. 75: 947-982. Kurtin WE and JA Zulich (1978) "Action spectrum for oxygen-dependent near-ultraviolet induced corneal damage." Photochem. Photobiol. 27: 329.
Kurzel R, ML Wolbarsht and BS Yamanashi (1977) "UV Radiation effects on the human eye." Photochem. Photobiol. Rev. 2: 133-167.
Kurzel RB (1973) "Trypotophan excited states and cataracts in the human lens." Nature 241:132-133.
Kuwabara T and S Okisake (1976) "Effect of electronic strobe flashlight on the monkey retina." Jap. J. Opthal. 20: 9-18.
Kuwabara T (1978 "Photic and photo-thermal effects on the retinal pigment ipithelium." In the Retinal Pigment Epithelium, Harvard University Press, Cambridge.
Kwan M, J Ninikoski and TK Hunt (1972) "In vivo measurements of oxygen tension in the cornea, aqueous human and anterior lens of the open eye." Invest. Opthal. 11: 108-111.
Lakhanpthal V et al. (1982) Arch Opthalmol 100: 1068-1070.
LaVail MM and BA Battelle (1975) "Influence of eye pigmentation and light deprivation on inherited retinal dystorphy in the rat." Exp. Eye Res. 21:167.
Lerman S (1980a) "Human ultraviolet light cataracts." Ophth. Res. 12: 303-314.
Lerman S (1980b) "Radiant energy and the eye." MacMillan Publishing Company, New York.
Lerman S (1982) "Ocular Photoxicity and PUVA therapy: An experimental and clinical evaluation." FDA Photochemical Toxicity Symp. J. Natl. Cancer Inst. 69: 287-302.
Lerman S (1983) "Psoralens and ocular effects in animals and man: In vivo monitoring of human ocular and cataneous manifestations." Conf. on Photobiologic, Toxicologic and Pharmacologic Aspects of Psoralens, March 1-3, 1982. J. Natl. Cancer Inst., In Print.
Lerman S and R Borkman (1976) "Spectroscopic evaluation and classification of the normal, againing and cataractous lens." Opthal. Res. 8: 335-353.
Lerman S, J Megaw, and K Gardner (1982a) "Psoralen-long-wave ultraviolet therapy and human cataractogenesis." Invest. Opthal. Vis. Sci. 23: 801-803.
Lerman S, J Megaw and K Gardner (1982b) "Allopurinol therapy and human cataractogenesis." Amer. J. Opthalmol. 94: 141-146.
Lerman S, JF Kuck, R Borkman and E Saker (1976) "Acceleration of an aging parameter (Fluorogen) in the ocular lens." Ann. Opthal. 8: 558-562.
Lerman S, JF Kuck, R Borkman and E Saker (1976) "Induction, acceleration and prevention (in vitro) of an aging parameter in the ocular lens." Opthal. Res. 8: 213-226.
Lerman S, JM Megaw, K Gardner, Y Takei and I Willis (1981) "Localization of 8-methoxyposoralen in ocular tissues." Opthal. Res. 13: 106-116.
Lerman S, K Gardner, J Megaw and R Borkman (1981) "The prevention of direct and photosensitized UV radiation damage to the ocular lens." Opthalmic Res. 13: 284-292.
Lerman S, J Megaw and I Willis (1980) "The photreactions of 8-methoxypsoralen with tryptophan and lens proteins." Photochem. Photobiol. 31: 235-242.
Leske MC and RD Sperduto (1983) "The epidemiology of senile cataracts: a review," American Journal of Epidemiology. 118: 152-165.
Lowenstein A and J Steel (1941) "Macular burning and ring scotoma." Glasgow Med. J. 135: 73-85.
Maher EF (1978) "Transmission and absorption coefficients for ocular media of the rhesus monkey." Report SAM-TR-78-32, USAF School of Aerospace Medicine, Brooks AFB, TX.
Marcantonio JM, G Duncan, PD Davis, and AR Bushell (1980) "Classification of human senile cataracts by nuclear color and sodium content." Exp. Eye Res. 31: 227-237.
Marshal, J (1970) "Thermal and mechanical mechanisms in laser damage to the retina." Invest. Opthalmol. 9: 97- 115.
Mao W and T Hu (1982), "An Epidemiologic Survey of Senile Cataract in China," Chinese Medical Journal, 95(11): 813-818.
Martinez GS, AJ Campbell, J Reiken and BC Allan (1982) "Prevalence of ocular disease in a population study of subjects 65 years and older." Am. J. Opthalmol. 94: 181.
McCanna, P. et al (1982) Arch Opthalmol 100: 1071-1073.
McFaul PA (1969) "Visual prognosis after solar retinopathy." Brit. J. Opthalmol. 53: 534-541.
Megaw J, J Lee and S Lerman (1980) "NMR analyses of tryptophan-8-methoxypsoralen photoreaction products formed in the presence of oxygen." Photochem. Photobiol. 32: 265-269.
Moss SH and KC Smith (1981) "Membrane damage can be a significant factor in the inactivation of E. Coli by near UV radiation." Photochem. Photobiol. 32: 203-210.
Naidoff MA and DA Sliney (1974) "Ritinal injury from a welding arc." Am. J. Opthal 77: 663-668.
Owsley C, DW Kline, JS Werner, V Greenstein and J Marshall (1986) "Optical radiation effects on aging and visual perception." In Light Toxicity. M Wahler and VM Hitchens (eds.). CRC Press, Boca Raton, Florida.
Parrish JS, RR Anderson, R Urback and D Pitts (1978) UV-A Biological Effects of Radiation with Emphasis on Human Responses to Longwave Radiation. Plenum Press, New York.
Pathak MA, DM Kraemer and U Guengerich (1972) "Formation of thymine dimers in mammalian skin by UV radiation in vivo" Photochem. Photobiol. 15: 177-185.
Pirie A (1968) Invest. Opthalmol. 7: 634-649.
Pirie A (1972) "Photo-oxidation of Proteins and Comparisons of Photo-oxidized Proteins with Those of the Cataractous Human Lens." Israeli J. Med Sci. 8: 1567-
Pirie A and KJ Dilley (1974) "Photooxidation of N-formylkynurenine and tryptophan peptides by sunlight or simulated sunlight." Photochem. Photobiol. 19: 115-118.
Pitts DG (1970) "A comparative study of the effects of ultraviolet radiation on the eye." Am. J. Optom. 47: 535-546.
Pitts DG (1973) "The ocular ultraviolet action spectrum and protection criteria." Health Physics 15: 559-566.
Pitts DG (1974) "The human ultraviolet action spectrum." Am. J. Optom. and Physiol. Optics 51: 946-960.
Pitts DG (1978) "The ocular effects of ultraviolet radiation." Amer. J. Optom and Physiol. Optics 55: 19.
Pitts DG (1981) "The threat of ultraviolet radiation to the eye - how to protect against it." J.Amer. Optom. Assn., 52: 949-957.
Pitts DG, PD Hacker and WH Parr (1977) "Ocular ultraviolet effects from 295 and 400 nm in the rabbit eye." DHEW (NIOSH) Publication no. 77-175.
Pitts DG, JPG Bergmanson and LWF Chu (1983) "Rabbit eye exposure to broad-spectrum fluorescent light.: Acta Ophthalmol (Copenhagen) Supp. 159.
Pitts DG and RB Kay (1969) "The photoophthalmic threshold for the rabbit.- Am. J. Optom. 46: 561-572.
Pitts DG and AP Cullen (1981) "Determination of infrared radiation levels for acute ocular cataractogenesis." Graefes Arch. Klin. Exp. Ophthalmol. 217:285-
Pitts DG and TJ Tredici (1971) "The effects of ultraviolet on the eye." Am. Ind. Hyg. Assoc. J. 32: 235-246.
Pitts DG, et al. (in press), "Optical Radiation and Cataracts," in Vicusal Health and Optical Radiation, M. Waxler and V. Hitchens, eds., CRC Press, Inc., Boca Raton, Florida.
Rapp LM, JG Jose and DG Pitts (1985) "DNA reapir synthesis in the rat retina following in vivo exposure to 300 nm radiation." Invest. Ophthalmol. Vis. Sci. 26: 384.
Ruffolo JJ Jr, WT Jr Ham, HA Mueller and JE Miller (1984) "Photochemical lesions in the primate retina under conditions of elevated blood oxygen." Invest. Ophthalmol. Vis. Sci. 25: 893-898.
Satoh K, M Bando and A Nakajima (1973) "Fluorescence in human lens." Exp. Eye Res., 16: 167-172.
Schmidt RE and JA Zuclich (1980) "Retinal lesion due to ultraviolet laser exposure." Invest. Ophthalmol. Vis. Sci. 19: 1166-1175.
Serafino GM and Frederick JE (in press) "Global modeling of the ultraviolet solar flux incident on the biosphere," prepared for the U S Environmental Protection Agency, Washington, D.C.
Sliney, D and M Wolbarsht (1980) Safety with Lasers and Other Optical Sources. Plenun Press, New York.
Spector A, D Roy and J Stauffer (1975) "Isolation and characterization of an age-dependent polypeptide from human lens with non-tryptophan fluorescence." Exp. Eye Res. 21: 9-24.
Spector A, O Roy and J Stauffer (1975) "Isolation and characterization of an age-dependent polypeptide from human lens with non-tryptophan fluorescence." Exp. Eye Res. 21: 9-24.
Spector A and WH Gardner (1982) "Hydrogen perioxide and human cataract." Exp. Eye Res. 33: 673-681.
Sprott GD, WG Martin and H Schneider (1976) "Differential effects of near UV and visible light on active transport and other membrane process in E. coli. Photochem. Photobiol. 24: 21-27.
Streeten BW and J Eshaghian (1978) "Human posterior subcapsular cataract: a gross and flat preparation study." Arch. Ophthal. 96: 1653-1658.
Sykes SM, WG Jr Robinson, M Waxler and T Kuwabara (1981) "Damage to the monkey retina by broad-spectrum fluorescent light. "Invest. Ophth. Vis. Sci. 20: 425-434.
Taylor HR (1980) "The Environment and the Lens" Brit. J. Opthal. 64: 303-310.
Thomas DM and KL Schepler (1980) "Raman spectra of normal and ultraviolet-induced cataractous rabbit lens." Invest. Opthalmol. Vis. Sci. 19: 904-912.
Trevithick JR, MO Creighton and WM Ross (1980) "Vitamin E prevents cataractogenesis in diabetic rats." Invest. Ophth. and Vis. Sci. (Suppl.) 19: 207-208.
Truscott RJW and RC Augusteyn (1977) "Oxidative changes inhuman lens protein during senile nuclear cataract formation." Biochem. Biophys. Acta 492: 43-52.
Truscott RJW, K Faull and RC Augusteyn (1977) Opthal. Res. 9: 263-8.
Turro NJ and A Lamola (1977) Photochemistry in the Science of Photobiology. KC Smith (ed), Plenum, New York, pp. 63-86.
Tso MOM and BJ Woodford (1983) "Effect of photic injury on the retinal tissues." Opthalmology 90: 952.
Tyrell RM (1976) Photochem. Photobiol. 23: 13-20.
Van Heyningen R (1971) Nature 230: 393-394.
Van Heyningen R (1973) Exp. Eye Res. 17: 137-147.
Van Heyningen R (1976) "What happens to the human lens in cataract." Sci. Amer. 233: 70-81.
Varma SD (1981) "Superoxide and lens of the eye: a new theory of cataractogenesis." Inter. J. quantum Chem. XX: 479-484.
Varma SD, a Tina and RD Richards (1977) "Protection against superoxide radicals in rat lens." Opthal. Res. 9: 421-431.
Varma SD, D Chand, YR Sharma and JF Kuck (1983) "Effect of vitamin E on cataract development in emory mice." Invest. Opthal. Vis. Sci. (ARVO Suppl. 24: 31.
Varma SD, D Chand, YR Sharma, JF Kuck and RD Richards (1983) "Oxidative stress on lens and cataract formation: role of light and oxygen." Presented at the 1st International Symposium on Light and Oxygen Effects on the Eye. Current Eye Research, In press.
Varma SD, NA Beachy and RD Richards (1982) "Photoperoxidation of lens lipids: prevention by vitamin E. Photochem. Photobiol. 36: 623-626.
Varma SD, S Kumar and RD Richards (1979) "Light-induced damage to ocular len cation pump: prevention by vitamin C." Proc. Natl. Acad. Sci. 76: 3504-3506.
Varma SD, VK Srivastava and RD Richards (1982) "Photoperoxidation in lens and cataract formation: preventative role of superoxide dismutase, catalase and vitamin C." Opthal. Res. 14: 167-175.
Warner, JS (1982) "Development of scotopic sensitivity and the absorption spectrum of the human ocular media." J. Optical. Soc. Am. 72: 247-258.
Waxler, M. (in press), "Long-Term Visual Health Problems: Optical Radiation Risks," in Visual Health and Optical Radiation, M. Waxler and V. Hitchens, eds., CRC Press, Inc., Boca Raton, Florida.
Weale RA (1982) "The age variation of senile cataract in various parts of the world." Br. J. Opthalmol. 66: 31-
Webb RB and MJ Peak (1981) "The effect of stray light in the 300-320 nm range and the role of cyclobutyl pyrimidine dimers in 365 nm lethality." Mutat. Res. 91: 177-182.
Weiter JJ and ED Finch (1975) "Matters arising: UV light and human cataract." Nature 254: 536-537.
Weiter JJ and S Subramanian (1978) "Free radicals produced in human lenses by a biophotonic process." Invest. Opthal. Vis. Sci. 17: 869-873.
Wiesinger H et al. (1956) "Transmission of light through the ocular media of the rabbit eye." Am. J. Opthalmol. 42: 907-910.
Williams, TP and BN Baker eds. (1980) The Effects of Constant Light on Visual Processes. Plenum Press, New York.
Wolbarsht, ML (1976) "The function of intraocular color filters." fed. Proc. 35: 44-50.
Wolbarsht ML, GS Goerge, WA Jr. Shearin, JA Klystra and MB III Landers (1983) "Retinopathy of Prematurity: A new look at an old disease." Paper presented at 1st Int. Symp. on Light and Oxygen Toxicity, Baltimore.
Wolfe E (1949) "Effects on visual threshold of exposure to radiation below 4000 angstroms." Trans Am. Acad. Opthalmol. Otolaryrgol. 53; 400-414.
Yoakum G, W Ferron, A Eisenstark and RB Webb (1974) "Inhibition of replication gap closure in E. coli by near UV photoproducts of L-tryptophan." J. Bacterial 119:62-69.
Young RW (1981) "A theory of central retinal disease." In Future Directions of Opthalmic Research. ML Sears (ed.). Yale University Press, New Haven, Conn.
Yu N-T, JFR Kuck and CC Askren (1979) "Red fluorescence in older and brunescent human lenses." Invest. Opthalmol. Vis. Sci. 18: 1278-1280.
Zigler JS and JD Goosey (1981) "Aging of protein molecules: lens crystallins as a model system." Trends in Biochem. Sci. 6: 133-136.
Zigler JS and JD Goosey (1981) "Photosensitized oxidation in the ocular lens: evidence for photosensitizers endogenous to the human lens." Photochem. and Photobiol. 33: 869-874.
Zigler JS, JB Sidbury, BS Yamanashi and M Wohlbarsht (1976) "Studies on brunescent cataract. I. Analysis of free protein-bound amino acids." Ophthal. Res. 8: 379-387.
Zigman S (1970) "Near-UV light and cataracts." Photochem. and Photobiol. 26: 437-441.
Zigman S (1981) "Photochemical mechanisms in cataract formation." In Mechanisms of Cataract Formation in the Human Lens." G. Duncan (ed.). Publ. Acad. Press, Inc. London. Pp. 117-149.
Zigman S and R Vaughan (1974) "Effects of near-UV light on the lenses and retinas of mice." Invest. Ophth. Vis. Sci. 13: 462-465.
Zigman S, Griess, T Yulo and J Schultz (1983) "Ocular Protein alterations by near UV light." Exp. Eye Res. 15: 255-264.
Zigman S, M Datiles and E Torczynski (1979) "Sunlight and Human Cataracts." Invest. Opthal. Vis. Sci. 18: 462-467.
Zigman S, T Yulo and G Griess (1976) "Inactivation of Catalase by near-ultraviolet light and tryptophan photoproducts." Mol. Cell. Biochem. 11: 149-154.
Zuclich JA (1977) "Lenticular effects of near-UV laser radiation." In Research on the Ocular Effects of Laser Radiation. Part II. Technology Incorporated, Final Report, Contrast F41609-73-C-0017. USAF School of Aerospace Medicine.
Zuclich JA (1980) "Cumulative effects of near-ultraviolet induced corneal damage." Health Phys. 38: 833.
Zuclich JA and JS Connolly (1976) "Ocular damage induced by near-ultraviolet laser radiation." 15: 760-764.